Introduction to IDMP Compliance:
The International Organization for Standardization (ISO) has developed the Identification of Medicinal Products (IDMP) standards, which outline standardized definitions for identifying and describing medicinal products intended for human use. These standards are designed to streamline the exchange of medicinal product information in a reliable and consistent manner.
In the pharmaceutical industry, a single finished product can take on three distinct forms:
- The pharmaceutical product as administered,
- The authorized medical product, and
- The packaged product that ships to market.
This illustrates just one aspect of the complexity hence there is a need for a common language throughout the lifecycle of a medicinal product.
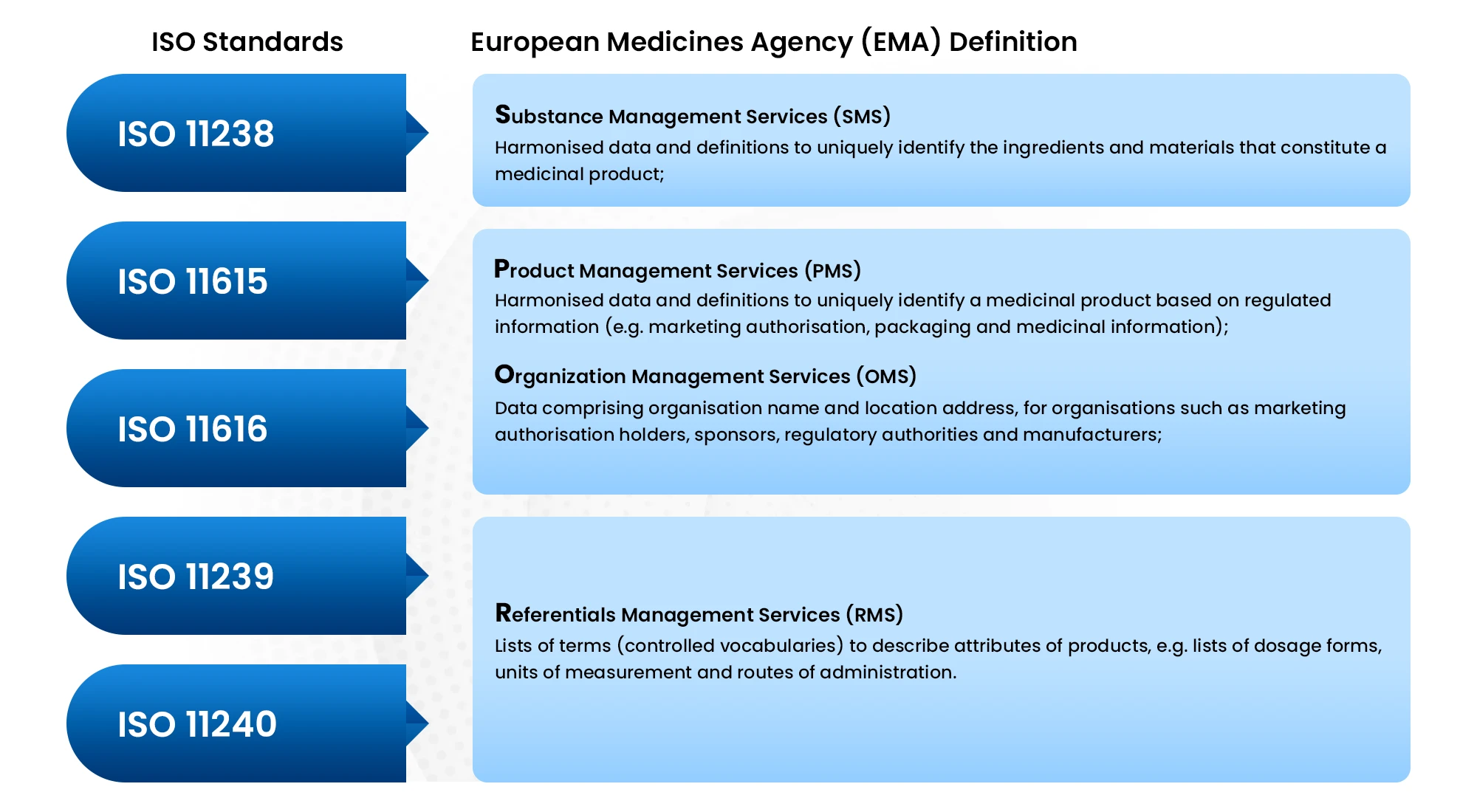
IDMP consists of five distinct standards, each of which defines concepts, data elements, and their structural relationships.
-
Substance Identification (ISO 11238)
Defines substances that constitute a medicinal product by their main, general characteristics. -
Medicinal Product Identification (ISO 11615)
Data elements that identify and characterize a medicinal product include the product name (authorized by regulatory agency), clinical particulars, pharmaceutical product (substance, dosage form, route of administration), medicinal product packaging, marketing authorization, manufacturer/establishment, etc. -
Pharmaceutical Product Identifier (ISO 11616)
Data elements that uniquely associate medical products with the same or similar pharmaceutical composition based on the following data elements: substance(s), strength(s) (units of measurement/presentation), reference strength(s), and dosage form. -
Dosage Form and Route of Administration (ISO 11239)
Data elements that provide information on pharmaceutical dose forms, units of presentation, routes of administration and packaging -
Units of Measurement (ISO 11240)
Data elements and structures for unique identification and exchange of units of measurement.
The European Medicines Agency (EMA) is implementing the ISO IDMP standards for the identification of medicinal products in a phased programme, based on the four domains of master data in pharmaceutical regulatory processes: substance, product, organization and referential (SPOR) data.
Challenges to IDMP Compliance:
- Complex data & processes: Pharmaceutical companies often handle extensive datasets spread across diverse systems, departments, and locations. This leads to the formation of data silos and inconsistencies. Achieving effective data management necessitates coordination and collaboration among regulatory authorities, various departments, and stakeholders.
- Technical Obstacles: Adopting IDMP standards entails the harmonization and standardization of data across various databases and systems. This task can prove particularly daunting, especially for organizations utilizing legacy systems or possessing diverse data structures. Overcoming these technical hurdles and ensuring seamless interoperability may require a significant amount of time and effort.
- Regulatory Constraints: Regulatory requirements evolve, necessitating continuous updates and adaptations to ensure ongoing compliance. Additionally, different regulatory authorities have different approaches to implementing IDMP standards.
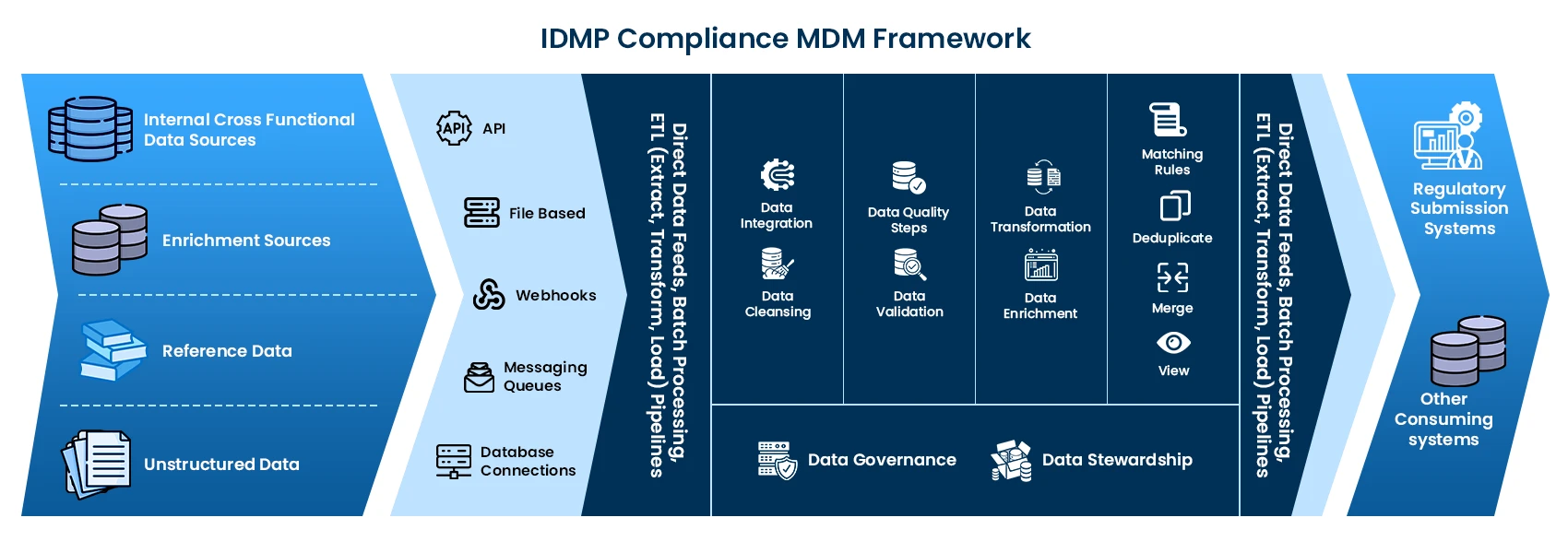
Why is MDM the solution for IDMP?
1. Data Standardization
- A medicinal product data is made up of data sets from various departments like regulatory, finance, quality, supply chain and many more. In addition to, large number of attributes that define a product, the same attribute takes multiple forms based on the source it is derived from.
- Standardization ensures that data is formatted consistently, making it easier to understand, compare, and integrate across various systems or organizations.
- MDM harmonizes disparate datasets, connecting to different data sources through seamless integration capabilities.
- MDM enables mapping and reconciling differences in terminology, classifications, or data models to establish common standards
2. Data Quality Management
- Product data can be available in structured and unstructured forms. Data may be entered manually or read from documents using Optical Character Recognition (OCR) technology. Multiple ways of ingesting data could lead to data quality errors.
- MDM incorporates data quality tools and processes to cleanse, enrich, and validate data, ensuring its accuracy, completeness, and conformity to regulatory standards.
3. Version Control and Audit Trail
- Medicinal product data keeps changing and updating throughout their lifecycle and not all attributes that define a product will be readily available on day 1 of its inception. For example, license number for a product can vary based on the country of registration and will be available only after the product is registered.
- Hence maintaining the audit of all changes and updates to the product data is essential for regulatory compliance.
- MDM tracks changes to data elements, maintains version control, and provides an audit trail, facilitating compliance documentation and regulatory reporting.
4. Data Stewardship & Data Governance
- IDMP mandates meticulous data management for medicinal products. So, efficient data governance is essential to uphold regulatory adherence, data integrity, and security while facilitating information exchange.
- Similarly, to ensure proactive management and oversight of an organization’s data assets, data stewardship will be critical.
- MDM provides a centralized platform for assigning data stewardship roles and responsibilities, facilitating oversight of data quality, integrity, and tools to enforce data standards, validate data accuracy, and resolve data conflicts.
5. Single Source of Truth
- IDMP compliance necessitates accurate and complete reporting of product data to regulatory authorities. Duplicacy can result in discrepancies in regulatory submissions, potentially leading to compliance issues, fines, or regulatory penalties.
- Inaccurate or inconsistent product data resulting from duplicacy can have serious implications for patient safety and public health.
- Core capability of MDM is to create a single source of truth for master data, by creating a unified, authoritative repository for medicinal product data ensuring consistency across domains.
Curious about how our MDM expertise can help pharmaceutical companies achieve IDMP compliance?
Contact Us
Error: Contact form not found.